ORIGINAL
Genetic diversity detection of the domestic horse (Equus caballus) by genes associated with coat color
Detección de la diversidad genética del caballo doméstico (Equus caballus) mediante genes asociados al color del pelaje
Luz Correa A,1* Biol, Cindy Reyes E,1 Biol, Enrique Pardo P,1 Ph.D, Teodora Cavadia M,1 M.Sc.
1Universidad de Córdoba, Facultad de Ciencias Básicas, Departamento de Biología, Grupo de Investigación Genes. Carrera 6 No. 76-103. Montería, Colombia.
*Correspondence: luzaraujo65@hotmail.com
Received: September 2014; Accepted: February 2015.
ABSTRACT
Objective. To assess the population structure and genetic diversity in populations of domestic horse (Equus caballus) in the municipality Cienaga de Oro-Córdoba (Colombia). Materials and methods. Random sampling were conducted between August and October 2013, in adult animals on farms seven districts, which was carried out phenotypic characterization of each animal, based on autosomal markers encoding morphological Extension (E) , Agouti (A), Cream (C), White (W), Gray (G), Tobiano (TO), Overo (O) and Roan (RN). Population genetic parameters: allele frequency, genetic diversity, gene flow, Hardy-Weinberg equilibrium and genetic distance were calculated through the program POPGENE 1.31; the genetic structure was assessed using the program FSTAT v. 2.9.3.2. Results. 341 individuals were analyzed in the seven populations studied, where the Extension gene Was the MOST faq frequently as the Overo and Tobiano genes showed the lowest values. Insignificant values of genetic variability and population recorded a global level, likewise, low genetic differentiation among populations, accompanied by a high gene flow was obtained; an excess of heterozygotes at population and global level was observed; to this is added the presence of Hardy-Weinberg equilibrium in all populations relative to the markers studied and low genetic distance values were reported. Conclusions. The populations are highly genetically related, a situation that may result from the existing geographical proximity between them, favoring genetic exchange and the establishment of a metapopulation.
Key words: Horse, Ciénaga de Oro, Córdoba, heterozygosity (Source: MeSH).
RESUMEN
Objetivo. Evaluar la estructura poblacional y la diversidad genética en poblaciones de caballo doméstico (Equus caballus), en el municipio Ciénaga de Oro, Córdoba (Colombia). Materiales y métodos. Se realizaron muestreos aleatorios entre los meses de Agosto y Octubre del año 2013, en animales adultos presentes en las fincas de siete corregimientos, donde se llevó a cabo la caracterización fenotípica a cada animal, atendiendo a los marcadores autosómicos de codificación morfológica Extension (E), Agouti (A), Cream (C), White (W), Gris (G), Tobiano (TO), Overo (O) y Roan (RN). Los parámetros genéticos poblacionales: frecuencia alélica, diversidad genética, flujo génico, equilibrio Hardy-Weinberg y distancia génica, fueron calculados a través del programa PopGene 1.31; la estructura genética se evaluó mediante el programa FSTAT v. 2.9.3.2. Resultados. Se analizaron 341 individuos en las siete poblaciones estudiadas, donde El marcador Extensión fue el de mayor frecuencia mientras los genes Overo y Tobiano presentaron los menores valores. Se registraron cifras poco significativas de variabilidad genética a nivel global y poblacional, así mismo, se obtuvo una escasa diferenciación genética entre las poblaciones, acompañado de un elevado flujo génico; se observó un exceso de heterocigotos a nivel poblacional y a nivel total, a esto se le suma la presencia de equilibrio Hardy-Weinberg en todas las poblaciones con relación a los marcadores estudiados y se reportaron valores bajos de distancia genética. Conclusiones. Las poblaciones se encuentran muy relacionadas genéticamente, situación que puede obedecer a la cercanía geográfica existente entre ellas, favoreciendo el intercambio genético y la constitución de una metapoblación.
Palabras clave: Caballo, Ciénaga de Oro, Córdoba, heterocigosidad (Fuente: MeSH).
INTRODUCTION
The domestic horse (Equus caballus) is a mammal of the Perissodactyla order and the Equiidae family; this species is characterized by having long and strong limbs, barrel type body covered by short hair, as well as a long neck that supports the moderately large head and a tail that extends until half the hindlimbs (1). This animal has been considered as one of the main domestic resources throughout human history, because its force, nobility and fidelity characteristics have been fundamental in the fight for freedom of mankind and the development of nations. Historically, Colombia has been a country with equine interest, and as such, there has been progress in understanding this important domestic resource with special emphasis in species related to the development of preventive sanitary and diagnostic schemes. However, there is a large gap in the genetic research field of the animal; therefore, a deeper study of this type should be conducted as a complement to the complete knowledge of the domestic horse (2).
The diversity of domestic species is considered an important component of the global biodiversity (3,4), specially the variability of zoogenetic resources, which is considered a key element of all production systems since it provides the raw material for genetic improvement and the adaptation of changing circumstances, being local breeds a natural and irreplaceable reservoir of genetic variability. Therefore, their understanding is key for the possible planning of long-term sustainable conservation strategies (5). This situation has been reflected in the undertaking of genetic population studies in local breeds cattle (6,7) and equine (8) in order to design management plans oriented towards their protection and improvement.
Phenotypic markers are a valuable tool when analyzing the genetic structure of populations due to their large data contents, easy manipulation and identification and fast results (9). Important studies in domestic populations of cats (9) and pigeons (10) have been conducted through the use of genes related to their coat color.
In light of the above, this research had the purpose of assessing the genetic degree of diversity and structure of populations in domestic horses (Equus caballus) in the municipality of Cienaga de Oro, Colombia.
MATERIALS AND METHODS
Study location. The study was conducted at the municipality of Cienaga de Oro (Cordoba), located at 8°53’ latitude north and 75°37’ latitude west of the Greenwich meridian. The town has an altitude of 13 m above sea level and an average temperature of 27°C, with an area of 751 km2, of which 368 km2 are in rural areas. The town includes the villages of Berastegui, El Siglo, Los Mimbres, Pijiguayal, Las Palmitas, Punta de Yañez and San Antonio del Tachira.
Obtaining of data. Random sampling was conducted between August and October 2013 in adult animals that are the property of the farms in each of the studied populations. Phenotypic characterization of each animal was conducted, based on encoding morphological autosomal markers (Extension, Agouti, Cream, Gray, White, Tobiano, Overo, Roan). Lastly, photographic records were taken of each individual.
Coat markers.
Extension (E). This gene originates the black color in the horse when it is expressed in a dominant form (E), while in the recessive condition (e), the coat does not acquire this color (11).
Agouti (A). In this dominant condition (A), the gene causes the black coloring to extend to the hair, the tail and the lower part of the legs; in a recessive (a) form it does not cause any change in the horse’s color (11).
Cream (C). The dominant gene (C) causes the solid color to dilute, which causes the color to clarify; while in its recessive presentation (c), it does not cause the color to dilute (11).
Gray (G). The dominant characteristic of the gene causes (G) to mix the white and black hair, giving a gray image; in its recessive condition (g), the horse does not show this coat color (11).
White (W). When the (W) gene appears the horse is totally white. This marker should be in a heterozygote condition (Ww) since the homozygosis is lethal; while in its recessive trait (w), the horse can have another color (11,12).
Tobiano (TO). In the dominant condition (TO), the horse has a series of white spots that cross the dorsal region until the belly, and even on the legs; in its recessive condition (to), this characteristic is not present (11).
Overo (O). Given that the (O) gen is dominant, white spots are present from the ventral region towards the dorsal region; the spots are usually small and can be present on the legs and the case; in its recessive form (o) this gene does not cause changes in the normal color of the horses (11).
Roan (RN). The horse that carries the dominant gene (RN) presents in the body a mix of white hairs in any base color, except on the hair and the legs; in its recessive condition (rn), this characteristic is not present (11).
Statistical design. The estimation of allele frequency of each marker at a population and at a global level, as well as the genetic diversity measures established by Nei corresponding to the expected heterozygosis (He), expected heterozygosis of the total population (HT), genetic differentiation coefficient (GST), genetic flow (Nm), Hardy-Weinberg equilibrium and genetic distance among the populations, were estimated with the PopGene 1.31 program (13). The genetic structure of the population, observing the fixation indexes proposed by Wright: FIS, FIT and FST, was calculated using the FSTAT v. 2.9.3.2 program (14).
The dendrogram that represents the estimated values of the genetic distance was elaborated with the UPGMA (Unweighted Pair Group Methodwith Arithmetic Mean) method using the MEGA 5,2 program (15).
RESULTS
In the seven population studies a total of 341 individuals were analyzed and distributed as follows: Berástegui (n=68), El Siglo (n=67), Pijiguayal (n=54), Punta de Yánez (n=47), Las Palmitas (n=46), Los Mimbres (n=41) and San Antonio del Táchira (n=18).
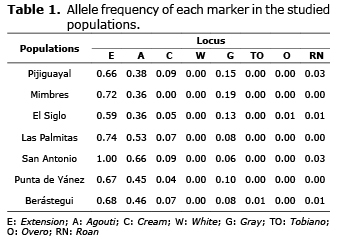
Allele frequencies. When calculating allelic frequencies for each population (Table 1), the Extension gene was the most frequent, especially in municipalities like San Antonio (p=1.000), Las Palmitas (p=0.744) and Los Mimbres (p=0.729); in the first two municipalities the Agouti gene frequency was significant, being the second marker in terms of frequency. On the other hand, the White marker, responsible for the white coat, was not found in any municipality; while the Tobiano, Overo and Roan markers were found in some municipalities in very low frequencies. On the other hand, Los Mimbres was the area with the less number of genes reported (only 3 and 8) followed by Las Palmitas and Punta de Yánez, which presented only half the reported genes; while that El Siglo had the most number of markers.
At global level, the Extension gene was the marker with the most frequency, followed by Agouti and Gray (Table 2); by contrast, the Cream, Tobiano, Overo and Roan genes presented the markers with the least frequency.
Genetic diversity. In each one of the populations, the medium level of genetic variability was low (Table 3), being San Antonio the municipality with the least heterozygosis (Hand=0.0958). On the other hand, at the marker level, El Siglo and Pijiguayal were the municipalities that resulted having the largest diversity indexes with respect to the Extension gene. However, for the Agouti marker, Las Palmitas was the area with the largest degree of genetic diversity at a general level by obtaining a value of 0.4977, followed by Berástegui (Hand=0.4964) and Punta de Yánez (Hand=0.4958) for the marker previously mentioned, which also presented the highest levels of genetic variability in all municipalities, except in El Siglo. Altogether, the Agouti marker presented the largest genetic diversity value followed by the Extension plan, whose index was significantly higher than the indexes registered by the rest of the markers (Table 4). On average, the genetic variability level on the total population was 0.1542. With respect to the Hardy-Weinberg equilibrium test, at a general level the municipalities reported equilibrium. This also happened for all markers.
Genetic differentiation and genic flow. The genetic differentiation level in Cienaga de Oro was very low (0.0452), which indicates that approximately 4.5% of the variation detected is due to differences between the populations; therefore, the seven populations were not significantly different for the studied markers and suggest that all these are considered as a single population.
In contrast, the higher value of the genetic flow (10.5569) indicates that the populations keep a considerable degree of genetic exchange, assuming a total of ten migrants per generation. Also, the number obtained is higher than 1 and 4, which indicates that the populations behave like a metapopulation.
Population structure. The negative numbers in each marker and in average (-0.0733) for the FIS coefficient (Table 5), show an excess of heterozygotes of the individuals with respect to each population and therefore, the absence of consanguinity is assumed. With values of -0.0064 for the Cream marker up to -0,3968 for the Tobiano gene, in relation to the FIT statistical, the average value (- 0.0246) reflects an excess of heterozygotes of the individuals with respect to the total population, covering values of -0.026 to -0.3809 for the Gray and Overo markers, respectively. It is important to highlight that the average FIS and FIT values do not move away too much from zero; therefore, it is assumed that the global population is close to the Hardy-Weinberg equilibrium. On the other hand, the average FST value was low, which indicates the minor genetic differentiation that exists in other populations. It is worth noting that a similar value was recorded for the GST coefficient.
Genetic distance. The genetic distance between populations was low, being Punta de Yánez and Berástegui the closest populations (Table 6), while El Siglo and San Antonio were the populations with the highest genetic differentiation; the number was not very significant since it did not surpass 4%. In this context, San Antonio presented the highest genetic distance values in comparison with the rest of the populations.
Considering the reported genetic distance values in the dendrogram (Figure 1), it can be observed that Punta de Yánez and Berástegui were the populations that were the closest and to which Las Palmitas is associated to. In relation to Pijiguayal and El Siglo, there is a considerable proximity, and also, they have a close relation with Los Mimbres. On the other hand, San Antonio was the farthest population from the other populations, but with an insignificant distance value, which allows to deduce that the populations altogether are very related.
DISCUSSION
The elevated frequency of the Extension gene in all populations can be due to the hypothesis proposed by several authors (16), which is based on the fact that the allele variations that make more melanin colors (dark) possible, are favored in the comparison with those colors with lighter tones. Also, it has been demonstrated that the absorption capacity of calorie radiation is more frequent in animals with dark coat than in those animals with lighter coats (17). This allows assuming that darker horses are better adapted to prevailing conditions in tropical climates, considering that equines are animals that usually spend the most part of the day grazing. On the other hand, the absence of white horses could be due to a particular condition that characterizes the White marker, but in homozygosis it causes the death of the animal (12). This fact explains the substantial reduction in the number of individuals that carry the gene.
The presence of the majority of markers in El Siglo, shows a large variety of genes available in the area. This situation is possibly due to geographic proximity between the studied populations and the surrounding municipalities, which favored the movement of migrants from one population to another and therefore, a considerable genetic exchange. This would explain the reduced number of genes reported in Los Mimbres, since this is the only population that does not have a direct contact with border populations. It’s convenient to specify that even though the studied populations are in the Hardy-Weinberg equilibrium, the total absence of migration, mutation and selection cannot be assumed because the changes produced by these evolutionary forces in genetic frequencies take long periods of time. Therefore, to relate the adaptation and the coat color, as well as to highlight the importance of migration, are acceptable criteria in this research.
The low genetic diversity in each one of the populations and at a global level, together with a elevated genetic flow (Nm=10.5), reflects a close relationship that exists among populations since a high genetic exchange exceeds the effects of the genetic drift and prevents the local distinction (18). For this reason, the genetic differentiation degree between populations was low (GST=0.0452). Genetic diversity as well as its close relationship with the genetic flow level in populations also influences significantly in genetic distances (19). Therefore, the distance values will be low as the diversity between populations is low. This indicates a direct relationship between these variables, which indicates that the low genetic diversity levels found were determining on the distance values recorded.
The excess of heterozygotes obtained through different fixation indexes (FIS and FIT) in each one of the populations, indicates that these present an almost homogeneous structure, which is a fact that would be attributable to the high genetic flow among them since the existence of a high exchange of genes prevents endogamy events within the populations (20) and provokes a reduction of homozygotes genotype. Also, this would be related to the probable randomization of mating, a scenario expected in populations with Hardy-Weinberg equilibrium and that would facilitate the presence of null levels of consanguinity (21).
A crucial fact in terms of genetic distance is the size of the population since these two parameters have an indirect relation in which, as the population size decreases the higher the genetic distance with the rest of the populations (19). In this context, it could be said that San Antonio, the population with the smallest size, resulted in the most distance population from a genetic point of view. A possible response to the reduced number of recorded individuals is due to the topographic characteristics of the area, since this municipality is located in between a mountain chain with a woody vegetation, conditions that would limit the keeping of horses and considering that these horses usually graze in plain areas without exuberant vegetation (1). Resuming the issue of genetic distances, it is important to note that the distance model used in this study is based on Nei’s proposal were the variation is attributed mainly to mutation and genetic drift, assuming an equilibrium in the two evolutionary forces (19); however, since these are populations that belong to a same species and that are developed in a same geographic area, the mutational phenomena is considered, being the genetic drift as the main cause for differentiation (20) of which effects, as mentioned before, are reduced when there is a high degree of genetic flow among the populations.
On the other hand, the great genetic proximity between Punta de Yánez and Berástegui corresponds to Lara et al (22) proposals in their work Psychotria acuminata, in which the genetic distance resulted to be directly proportional to the geography, since the populations mentioned herein are very close geographically, this situation is similar to Las Palmitas, which was closely related to the previously mentioned populations, specially with Berástegui, of which is very close in spatial terms; for Pijiguayal and El Siglo, although not close geographically, they were close from a genetic point of view, which could be due to the massive movement of horses through the main highway that is an important point of connection between both populations. It is worth remembering that this study was only conducted at a municipal level; therefore, a marked geographic distance as such cannot be considered since the most part of the genetic distance values does not exceed 1%, which would indicate that the populations behave as one.
The insignificant values of genetic variability, together with a high degree of genetic flow, allow to infer that the populations that are genetically very related and that behave as a metapopulation is situation that is attributed to geographic proximity of all the populations from a structural point of view and indicates the need to introduce new individuals from external populations, in order to increase the genetic diversity levels and to facilitate the heterogeneity in terms of the population’s structure.
REFERENCES
1. Álvarez-Romero J, Medellín RA. Equus caballus. Vertebrados superiores exóticos en México: diversidad, distribución y efectos potenciales. Instituto de Ecología, Universidad Nacional Autónoma de México; 2005.
2. Naranjo S. Evaluación citogenética del caballo criollo colombiano. [Tesis de Maestría]. Medellín: Facultad de ciencias, Universidad Nacional de Colombia; 2012.
3. Ajmone-Marsan P. A global view of livestock biodiversity and conservation - Globaldiv. Anim Genet. 2010; 41(Supl 1):1-5.
4. Boettcher P, Tixier-Boichard M, Toro M, Simianer H, Eding H, Gandini G, et al. Objectives, criteria and methods for using molecular genetic data in priority setting for conservation of animal genetic resources. Anim Gene 2010; 41(Supl 1):64-77.
5. Giovambattista G, Rogberg MA, Ripoli M, Villegas E, Díaz S, Posik D, et al. La genética molecular de bovinos y equinos criollos en los albores del siglo XXI. J Basic Appl Genet 2010; 21(Supl 1):1-14.
6. Piedrahíta A, Posso A, Muñoz J, Alvarez L. Variabilidad genética de Hartón del Valle mediante RAM. Facultad de Ciencias Agropecuarias, Universidad Nacional de Colombia; 2007.
7. Bejarano D, Pedraza A, Rocha J, Martínez R. Variabilidad genética en subpoblaciones comerciales de la raza criolla colombiana Romosinuano. Rev Corpoica 2012; 13(Supl 1):97-107.
8. Jimenez L, Cañon J, Sanchez C, Sandoval F, Gomez A. Análisis de la variabilidad genética en caballos criollos llaneros colombianos mediante la utilización de microsatélites. Rev Col Cienc Pec 2007; 20(4):573-574.
9. Ruiz-Garcia M, Alvarez D, Shostell J. Population genetic analysis of cat populations from Mexico, Colombia, Bolivia, and the Dominican Republic: identification of different gene pools in Latin America. J Genet 2005; 84(Supl 1):147–171.
10. Čanády A, Mošanský L. Population size and plumage polymorphism of feral pigeon (Columba livia) from urban environment of Košice city (Slovakia). J Zool & Ecol 2013; 23(2):104–110.
11. Bartolome E, Azor P, Gomez M, Peña F. La determinación genética del color de la capa en el caballo: Bases y aplicación al caballo de la raza Pottoka. Departamento de genética, Universidad de Córdoba (España); 2008.
12. Haase B, Brooks S, Schlumbaum A, Azor P, Bailey E, Alaeddine F, et al. Allelic heterogeneity at the equine KIT locus in dominant White (W) horses. PLoS Genet 2007; 3(11): 2101-2108.
13. Yeh FC, Yang RC, Mao J, Ye Z, Boyle TJ. POPGENE: the Microsoft Windows-based user-friendly software for population genetic analysis of co-dominant and dominant markers and quantitative traits. [Programa estadistico]. Version 1.31. Edmonton, Canadá: University of Alberta, Department of Renewable Resources; 1999.
14. Goudet J. FSTAT: a Program to estimate and test gene diversity and fixation indices. [Programa estadístico]. Version 2.9.3.2. Lausanne, Switzerland: University of Lausanne, Department of Ecology & Evolution; 2002.
15. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA 5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 2011; 28(10):2731-2739.
16. Jacquin L, Haussy C, Bertin C, Laroucau K, Gasparini, J. Darker female pigeons transmit more specific antibodies to their eggs than do paler ones. Biol J Linne Societ 2013; 108(3):647-657.
17. Gomes da Silva R, Guilhermino M, Façanha de Morais D. Thermal radiation absorbed by dairycows in pasture. Int J Biometeorol 2010; 54(Supl 1):5-11.
18. Piñero D, Barahona A, Eguiarte L, Rocha A, Salas R. La variabilidad genética de las especies: Aspectos conceptuales y sus aplicaciones y perspectivas en México. Conabio 2008; 1(Supl 1):415-435.
19. Demarchi D. Microsatélites, distancias genéticas y estructura de poblaciones nativas Sudamericanas. Rev Arg Antropol Biol 2009; 11(Supl 1):73-88.
20. Cortés O. Análisis de la variabilidad genética en la raza bovina de lidia utilizando información molecular. [Tesis doctoral]. Madrid: Universidad Complutense de Madrid; 2008.
21. Aranguren-Méndez J, Román R, Villasmil Y, Chirinos Z, Romero J. Componentes de varianza y parámetros genéticos para características de crecimiento en animales mestizos de doble propósito. Rev Científica FCV-LUZ 2006; 16(1):55-61.
22. Lara A, Valverde R, Rocha O, Gómez L. Variabilidad y diferenciación genética en cuatro poblaciones de la planta medicinal Psychotria acuminata en Costa Rica. Agron Costarr 2003; 27(2):29-42.